Introduction: β-thalassemia is a genetic blood disorder characterized by ineffective erythropoiesis and anemia. Although RBC transfusions are a key supportive treatment for patients (pts) with anemia due to β-thalassemia, they may be associated with life-threatening complications including iron overload. Luspatercept, a first-in-class erythroid maturation agent, is approved by the FDA for the treatment of anemia in adult pts with β-thalassemia requiring regular RBC transfusions.
Here we present a longitudinal analysis of the benefits of luspatercept on RBC transfusion burden (TB) in the BELIEVE trial, a phase 3, double-blind, randomized, placebo (PBO)-controlled study evaluating the efficacy and safety of luspatercept in adult pts with β-thalassemia requiring regular RBC transfusions (NCT02604433; Cappellini MD, et al. N Engl J Med 2020;382:1219-31).
Methods: Pts were aged ≥ 18 y with β-thalassemia or hemoglobin (Hb) E/β-thalassemia (compound β-thalassemia mutation and/or multiplication of α-globin genes was allowed) and required regular RBC transfusions (6-20 RBC units in the 24 wks prior to randomization, no transfusion-free period > 35 days). Pts were randomized 2:1 to luspatercept 1.0 mg/kg (up to 1.25 mg/kg allowed) or PBO subcutaneously every 3 wks for ≥ 48 wks. Pts in both treatment arms continued to receive best supportive care, including RBC transfusions to maintain target pretransfusion Hb levels and iron chelation therapy. After study unblinding, pts randomized to PBO were eligible to cross over to receive luspatercept in an open-label phase.
Mean RBC units transfused and mean change in RBC TB and number of visits were assessed in luspatercept (by primary endpoint responders and non-responders) and PBO arms during the first 48 wks. Long-term changes in RBC TB and visits in luspatercept pts remaining on treatment were assessed every 24 wks from treatment initiation to data cutoff (July 1, 2019).
Results: Of 336 pts enrolled, 224 were randomized to the luspatercept arm and 112 to PBO. During the 24 wks prior to randomization, median RBC TB was 14.3 RBC units (range 6.0-26.0) and median pretransfusion Hb level was 9.27 g/dL (range 4.5-11.7). As of July 1, 2019, median treatment duration for pts in the luspatercept and PBO (prior to crossover) arms was 119.1 and 74.7 wks, respectively. 68.2% of pts initially randomized to the luspatercept arm were still receiving treatment at the end of 2 y.
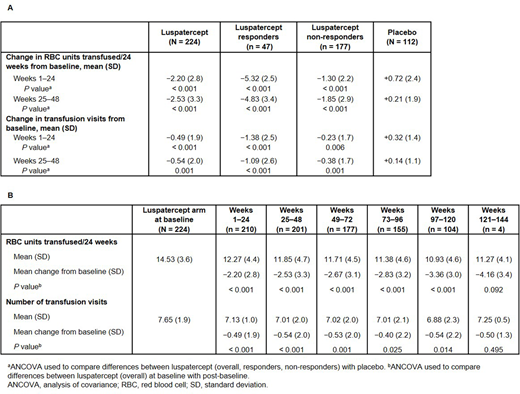
Pts in the luspatercept arm experienced a mean change of −2.20 RBC units/24 wks transfused vs +0.72 RBC units/24 wks in PBO-treated pts during Wks 1-24 compared to baseline (least squares [LS] mean difference −2.95; 95% confidence interval [CI] −3.59, −2.32; P < 0.001) (Table A). In Wks 25-48, mean changes of −2.53 and +0.21 RBC units/24 wks transfused were reported in luspatercept- and PBO-treated pts, respectively (LS mean difference −2.76; 95% CI −3.46, −2.06; P < 0.001). During Wks 1-24 and 25-48, luspatercept responders (defined as pts achieving ≥ 33% reduction in RBC TB during Wks 13-24, with a reduction of ≥ 2 RBC units, vs baseline) experienced mean transfusion reductions of −5.32 and −4.83 RBC units/24 wks, respectively, and luspatercept non-responders experienced mean changes of −1.30 and −1.85 RBC units/24 wks. Luspatercept-treated pts continued to experience durable, sustained reductions in RBC units up to 144 wks of follow-up (Table B).
During Wks 1-24, luspatercept-treated pts experienced a mean change of −0.49 in transfusion event frequency vs +0.32 for PBO-treated pts (LS mean difference −0.78; 95% CI −1.16, −0.40; P < 0.001) (Table A). Mean changes in transfusion visits of −0.54 and +0.14 were experienced by pts in the luspatercept and PBO arms, respectively, during Wks 25-48 (LS mean difference −0.65; 95% CI −1.03, −0.26; P = 0.001). Luspatercept responders and non-responders reported mean reductions in transfusion visits during Wks 1-24 (−1.38, P < 0.001 and −0.23, P = 0.006, respectively) and Wks 25-48 (−1.09, P < 0.001 and −0.38, P = 0.010, respectively) vs baseline. Sustained reductions in transfusion visits persisted for over 2 y; as the BELIEVE trial is still ongoing, only a small number of pts could be evaluated at later time points (Table B).
Conclusions: Luspatercept was associated with sustained reductions in RBC transfusion units and visits in responders and non-responders during the first 48 wks vs PBO. Pts on luspatercept continued to experience reductions in RBC TB and events over 2 y.
Taher:BMS: Consultancy, Research Funding; Ionis Pharmaceuticals: Consultancy; Vifor Pharma: Consultancy, Research Funding; Silence Therapeutics: Consultancy; Novartis Pharmaceuticals: Consultancy, Research Funding. Viprakasit:Agios Pharmaceuticals, Ionis Pharmaceuticals, La Jolla Pharmaceuticals, Protagonist Therapeutics, Vifor Pharma: Consultancy, Research Funding; BMS, Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hermine:Roche: Consultancy; Celgene BMS: Consultancy, Research Funding; AB Science: Consultancy, Current equity holder in publicly-traded company, Honoraria, Patents & Royalties, Research Funding; Alexion: Research Funding; Novartis: Research Funding. Porter:Protagonist Therapeutics: Honoraria; Vifor Pharmaceuticals: Honoraria; Silence Therapeutics: Honoraria; La Jolla Pharmaceuticals: Honoraria; Agios Pharmaceuticals: Consultancy, Honoraria; BMS: Consultancy, Honoraria; bluebird bio, Inc.: Consultancy, Honoraria. Piga:BMS: Research Funding; Novartis: Research Funding. Kuo:Pfizer: Consultancy, Research Funding; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Apellis: Consultancy; Bluebird Bio: Consultancy. Coates:Celgene, BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sangamo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios pharma: Consultancy, Honoraria; apo pharma (Chiesi Pharma): Consultancy, Honoraria; Vifor Pharma: Consultancy, Honoraria. Voskaridou:ACCELERON Company: Consultancy, Research Funding; BMS: Consultancy, Research Funding; PROTAGONIST Company: Research Funding; ADDMEDICA Company: Consultancy, Research Funding; NOVARTIS Company: Research Funding; GENESIS Company: Consultancy, Research Funding. Kattamis:Agios: Consultancy; Ionis: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Vifor: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apopharma/Chiesi: Honoraria, Speakers Bureau; Genesis Pharma SA: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Shetty:BMS: Current Employment, Current equity holder in publicly-traded company. Zhang:BMS: Current Employment. Tian:BMS: Current Employment. Miteva:BMS: Current Employment. Zinger:Celgene International, A Bristol-Myers Squibb Company: Current Employment. Tang:BMS: Current Employment, Current equity holder in publicly-traded company. Backstrom:Acceleron Pharma: Current Employment, Current equity holder in publicly-traded company; BMS: Current equity holder in publicly-traded company. Cappellini:BMS: Honoraria; CRISPR Therapeutics, Novartis, Vifor Pharma: Membership on an entity's Board of Directors or advisory committees; Genzyme/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal